A Mid-Twentieth Century Start Date for Anthropocene Geology
There is an astounding synchronism between the onset of the Anthropocene and the emergence of the technical means of registering and understanding it. Science historian Christoph Rosol sketches out the marriage of paleoceanography with isotope chemistry that took place in the middle of the twentieth century, highlighting the interdisciplinary co-evolution of an epistemic practice that sits at the heart of contemporary chronostratigraphy.
Paleoceanography …
At the beginning of the study of the ocean’s history, the ocean didn’t have one. For a long time, the pelagic deep sea was unfathomable. Sounding the bottom of near-coastal waters for navigational purposes and examining the type and color of the ooze, silt, or sand that stuck to the plumbing line was common seafaring practice since at least the time of Herodotus.1 Yet a regular sounding line was quite useless in the open sea, where heavy currents and the enormous depths defeated any attempts at measurement, let alone sediment recovery. The first controlled effort to sample deep-sea sediments was undertaken in the mid-nineteenth century to assess whether the sea floor was suitable to safely store telegraphic cables.2 Essentially, it consisted of a metal pipe inserted into a heavy cannonball. The few inches of ooze stuck in the pipe-cannon ball apparatus was proof enough for the crew that the sounding had indeed been successful. But even long after the pioneering Challenger expedition of 1872–1876, oceanographers generally believed that any organic material in the open ocean just deposited in a more or less constant fashion. Like never-ending snowfall, the time-honored yet history-less organisms who live and die in the water just sink to the sea floor, which accumulates great layers of monotonic material over geologic periods.
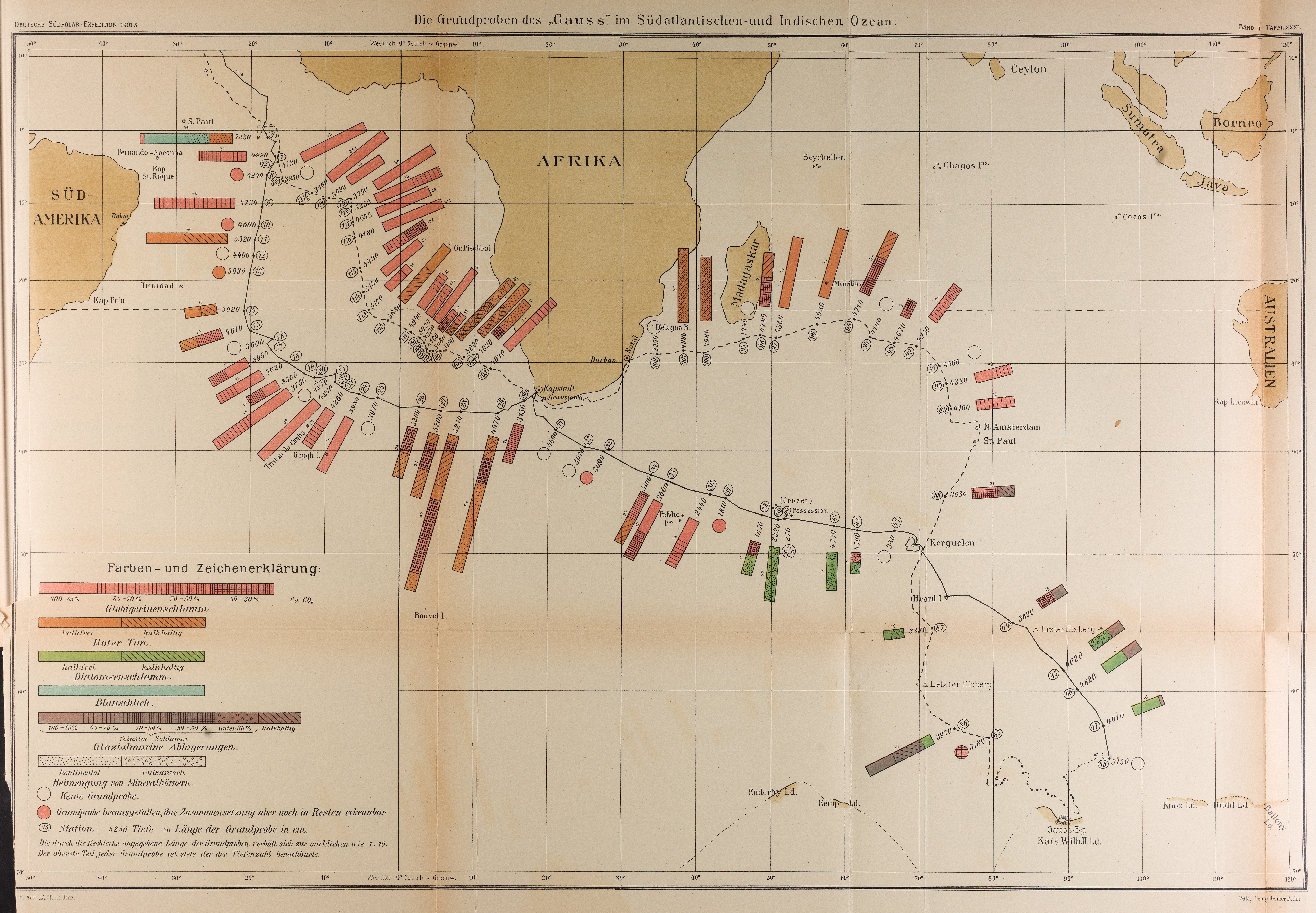
The assumption of the ocean’s timelessness was largely due to the fact that early oceanographers were only able to extract relatively small and hence unstratified sediment samples from the deep bottom sea floor. It was not until the Gauß research expedition made its way into the Southern Ocean (1901–1903) that this view changed. The particular equipment used by the Gauß crew, so-called “Bachmann’sche Schlammröhren” (“Bachmannian ooze pipes” or “corers”), allowed for an extraction of hitherto unseen cores of some 80 cm in length which finally showed a distinct layering. From then on, sediment cores showing no colored changes, no “history,” became the rare exception.3 The Jena-based geologist Emil Philippi wrote:
Krümmel assumed for the few cases known to him, in which Globigerina ooze was underlaid by red clay, a recent upward motion of the sea floor. […] Yet, the general distribution of normal calcareous layers suggests a climatic cause. […] The lower, less calcareous part of the subantarctic samples has probably been accumulated in a colder period, perhaps in a phase within the Quaternary ice age.4
Drilling sites of the “Gauß Expedition” of 1901–1903 in the Southern Atlantic and Indian Ocean. Emil Phillippi, Die Grundproben der Deutschen Südpolar-Expedition 1901–1903, vol. 2. Reimer Verlag, Berlin, 1912, © All rights original publisher
Later, in the early 1950s, the Swiss geologist Eugen Wegmann described this turn to a climatic explanation of sedimentary stratification (“Geschichtung”) as follows:
In the third stage [of polar research] the dynamic picture of space is integrated into a historical perspective. Each of the phenomena of the polar regions gets a history, a beginning, a growth, and an end, depending on the time frame. In this third stage, earlier methods are now accompanied by those methods that allow the traces of past events to be interpreted and defined chronologically. […]
Since Philippi realized that the samples of the German Antarctic expedition conveying two different types of sediment groups are witnesses of different climatic conditions […] a new archive has been made available: that of the sea floor.5
Wegmann’s words make it clear that the biostratigraphic method has been well established for quite a time already: once excavated, ooze showing strata of colored clay alternating with strata of foram shells was understood to analyzable in another place and context, the laboratory, under the interpretative assumption of having a decent indicator of paleoclimatic fluctuations at hand. In theory, climate change is a subject long explored; in practice, it was just lacking sufficient equipment.
Take, for instance, the German Meteor expedition of 1925–1927 over the equatorial Atlantic. The great achievement of this expedition was in finally mapping the Atlantic seabed, the “conversion of the hidden ‘nature’ of the deep ocean into stable and communicable profiles.”6 Yet the expedition’s coring efforts remained unsuccessful, often hauling up damaged cores that made a clear analysis of climatic layers impossible. It was not until the 1940s when the Swede Börje Kullenberg developed his “piston corer” that the recovery of non-distorted sediment cores was practically possible.7 This device, whose principle is still in use today, releases the weight with a remote trigger, allowing a deep penetration of the ocean floor. With the help of the piston corer, the Swedish Albatross Expedition (1947–1948) was able to capture sediments of up to 9 m in length.
A few years later, the German marine biologist Wolfgang Schott analyzed three of the Albatross cores.8 By identifying foram species, determining their quantity, and transferring that number onto an ordinary warm-cold axis, he obtained fluctuating curves that just needed to be geochronologically synchronized in order to show the alternation of the last glacial-interglacial cycles up to the Illinoian stage (ca. 300,000–130,000 years ago). Neatly correlated curves fluctuating between warm (w) and cold (k) were now derived from single-celled microorganisms, which thereby have been duly incorporated into the long-established circuit of paleontological “climate witnesses” (“Klimazeugen”), that is, climate proxies.
The painstaking procedure that produced such foraminifer quantities is described in Schott’s earlier account of methods and instrumentation aboard the Meteor. First, the relative percentage of different foram species contained in a filtered and heat-dried sample was determined by counting the shells under a microscope—between 400 and 600 in each sample.9 In a second step, the processed sample was weighed to arrive at approximate numbers of total species in one gram of original sample material. To calibrate this procedure, 4,000 microscopic shells were actually counted, patiently so, producing a total weight of just 0.17152 grams as reference standard.10 There is hardly any other empirical science besides micropaleontology that relies so much on precision instrumentation and a scientific culture of laborious counting.
Linear time series, variability, frequency: a true paleoceanography crystallized around the quantified analysis of certain microorganisms. “Paleoceanography deals with the history of the ocean.”11 It is the history of the fluid medium itself that is stored in the remnants of tiny shells. The medium continues to be the message: “In the composition of the community of the dead [Totengemeinschaft] the living community of pelagic foraminifers is still visible,” Schott wrote in another paper, while “among the physico-chemical factors of the environment the temperature of the seawater is probably the most crucial.”12 Apparently, the transfer function of single-celled climate witnesses (“Klimazeugen”) is to reconstruct the ancient state of the sea in the silence of its depth. This general approach to Earth’s history does not decisively change if one replaces a microscope with a mass spectrometer.
… marries geochemistry …
At the time Schott gave his paleoceanographic analysis, isotope geo-chemistry had already been established for a few years, adding a most potent while also spectacularly novel treatment to the already wide set of empirical techniques that constitute the study of paleoclimates, and geology more broadly.
Geochemistry, the study of the concentrations, distributions, and general cycling of elements on Earth, is essentially an old subject. It has an intimate relationship with agricultural and soil chemistry, but also with mineralogy and geology in a more direct sense. In fact, a variety of chemical experimental methods decisively contributed to the elaboration of geological theories in the seventeenth and eighteenth centuries, while also playing a decisive role in the neptunist-plutonist controversy in the nineteenth century.13 Basel electrochemist Friedrich Schönbein, who coined the term “geochemistry” in the 1830s, had already spoken of the potency of the chemist “to write the history of the globe” by comparing distinct chemical formations, while Karl Gustaf Bischof, author of the first textbook on geochemistry in the 1840s, regarded the Earth as a “vast chemical laboratory.”14
Nevertheless, a discussion around comparing of the distribution and migration of different isotopes of one and the same element through the atmosphere, the ocean, and Earth’s crust owes its possibility entirely to the laboratory demonstration of such nuclear varieties among naturally occurring radioactive elements by radiochemists since around 1910. Chemical elements possessing the same chemical and physical properties, but differing in atomic weights and radioactive properties (if radioactive), presented a burgeoning field of investigation and analytical techniques to study natural processes.
Given their name by Frederick Soddy in 1911, isotopes soon presented their suitability for a variety of methods. In the early twenties, George de Hevesy used radioactive isotopes to trace chemicals in plants and animals, thus already utilizing the circulation of isotopic species as an analytical tool for the study of living processes. No less important was the establishment of the radiometric dating method. By measuring radioactive decay of radionuclides with a known half-life, the absolute age of rock formations could be derived. The first measurements of uranium’s decay into lead that resulted in this method had already been pioneered, ante litteram, in 1907, and geochronological dating became highly fashionable in the following decades (see e.g. Hahn 1932). High-precision measurements of the isotopic ratio between Uranium-235 (235U) and Uranium-238 (238U), most notably by Alfred O. Nier in the mid-1930s, then finally cleared the way to putting geochronology on a firm quantitative basis.
The accuracy of Nier’s measurements was achieved by his own design of a much-improved mass spectrometer. In 1913, J. J. Thompson showed that ionized neon (that is, neon skimmed of its electrons) passing through a magnetic and electrical field produces two distinct paths on a photographic plate according to two different atomic masses, i.e. isotopic weights (20N and 22N). Since neon is a stable element, this was the first evidence of non-radioactive isotopes. The first reliable mass spectrographs based on the photographic principle were constructed in the late 1910s by Arthur Jeffrey Dempster and Thompson’s disciple Francis William Aston, the former presenting the basic design for all later developments. Besides the fact that Nier’s late 1930’s design obtained accurate mass spectra with relatively cheap material and thus allowed routine measurements, its specific importance was it could reliably measure lighter elements, most notably carbon.15 Nier could show how the isotopic abundance of 13C varies between different samples, though the origin of this natural separation process was unknown.
The final explanation for this process and its application to paleotemperature profiling was given by nuclear chemist Harold C. Urey. In 1931, Urey had shown how to separate, or “fractionate,” light from heavy hydrogen by a simple thermodynamic process, that is, distillation through careful warming: 1H evaporates more easily than 2H, leaving a condensate of heavy hydrogen, or “deuterium.” During the war, Urey worked on a combination of different separation processes—centrifugal separation, gaseous, and thermal diffusion—for uranium enrichment. The architecture of these apparatuses was essentially quite simple, given that it merely had to perform a cascading iteration of a physical separation process. However, many technical problems emerged, one of them lying in properly sealing the equipment.16
Setting up tent at the newly-founded Institute for Nuclear Studies at the University of Chicago in 1945/46, Urey then transferred his principle of fractionation theoretically to a variety of natural processes and chemical cycles. If 235U and 238U are separated by a thermal diffusion process within the laboratory, the same could also occur naturally with lighter elements, which are abundant in the geochemical composition of the Earth. This possibility implies that one could deduce a given temperature by studying the isotopic composition preserved in a geologic sample. It soon became clear that, theoretically, a precise measurement of the ratio between different oxygen isotopes could provide a sort of “paleothermometer”: “Accurate determinations of the 18O content of carbonate rocks could be used to determine the temperature at which they were formed.”17
In a 1948 lecture titled “Oxygen isotopes in nature and in the laboratory,” Urey summarizes the idea as follows:
The temperature coefficient for the abundance of the oxygen isotope in calcium carbonate makes possible a new thermometer of great durability, which may have been buried in the rocks for hundreds of millions of years after recording the temperature of some past geological epoch and then having remained unchanged to the present time. It is evident that, if an animal deposits calcium carbonate in equilibrium with the water in which it lives, and the shell sinks to the bottom of the sea and is buried securely […], it is only necessary to determine the ratio of the isotopes of oxygen in the shell today in order to know the temperature at which the animal lived.18
Thus, out of an isolated experimental system in an isolated (if not sealed-off) nuclear research facility grew a central geoscientific method based on the flow of elements in an open system: stable isotope geochemistry.
Nevertheless, as Urey noted, “the first problem in the application of this method to paleotemperatures is the construction and operation of very sensitive mass spectrometers.”19 The modifications and improvements of the Nier-type mass spectrometer were mainly to stabilize the ionization irradiator by a solid power supply, reliable amplifier valves, and better emission control modules.20 What determined the success or failure, here, were sophisticated electronics originating from war laboratories like the MIT Rad Lab. The multiplied precision needed to profit from the tiny temperature coefficient—essentially only 0.0000007 atomic weight units by a temperature change of 1°C—is highly symptomatic of the post-war shift from mechanical or optical apparatuses to the controlled application of electronics.21
Still, Urey’s first experiments with marine sediments encountered more mundane problems and initially turned out to be a “fiasco.”22 Different species, originating from different ecological zones within the water column, that is, benthic and pelagic organisms, were mixed in the fossilized layer of calcium carbonate. No meaningful paleotemperature could be derived from this conglomeration of different marine environments. To solve this problem of mixed fauna and to bring in micropaleontological knowledge and practice, Urey hired the Italian Cesare Emiliani for his Chicago lab. Emiliani’s painstaking work in separating the species eventually led to a breakthrough. Shortly after Urey had, together with his colleague Stanley Miller, proven that life on this planet could have originated from anorganic components (the famous Miller-Urey experiment), Emiliani was able to publish a paleothermometric graph that correlated with the glacial-interglacial cycles of the last 290,000 years, covering basically the same time period as Schott’s graph of three years earlier while applying a decisively different quantification method, stable isotope spectrometry.23 This method was subsequently refined, finally demonstrating the periodic fluctuations of ice volumes over millions of years that Milutin Milanković had already calculated and explained by orbital cycles some 25 years earlier.24
Gerald J. Wasserburg, Cesare Emiliani, and Harold C. Urey in their lab at the University of Chicago, ca. 1953. Courtesy University of Chicago Photographic Archive, Special Collections Research Center, University of Chicago Library
… and informs Anthropocene stratigraphy
Such is the admittedly very rough sketch of the fusion of nuclear chemistry and oceanography, and hence the beginning of the modern, ocean-based paleosciences that leap beyond classical land-based Quaternary geology. In a kind of perplexing about-face, current Anthropocene geology, as far as it is concerned with marine cores, is now only interested in these very first inches of ooze that appear at the top of the sampling column. The measurement and identification of the lower boundary of the Anthropocene relies on neither the sampling of the pelagic deep sea floor nor on exceptionally long cores. With the focus on only the most recent deposits, Anthropocene stratigraphy, as far as it concerns marine deposits, effectively goes back to the age-old practice of sounding near coastal waters and estuaries.
Still, the technical means and general episteme of registering the Anthropocene are to a large extent informed by the geochemical practice, that I tried to describe here in only a very rough manner and with a special focus on the paleotemperature profile. This practice requires a whole assemblage of material configurations—drilling vessels, hole re-entry cones, core repositories, and above all a highly sensitive mass spectrometers consisting of ionizer chambers, lenses, apertures, circuits, processors, but also reference material, chemical reagents, etc.—to mediate between what the marine geologist “sees” and what the laboratory geologist “understands” (while both are, of course, often times one and the same person). Without walking through this process of carefully recovering sediment cores, organizing their archival storage and registry, and analyzing them according to the highly sophisticated and calibrated methods of stable isotope and other forms of geochemistry, there is simply no modern chronostratigraphy. And without chronostratigraphy, the Anthropocene—as a unit of geological time—would remain elusive.
By fusing the old concept of the stratigraphic column as bearer of fossils and, hence, geological time, with the explanatory framework of elementary cycles, isotope chemists could invigorate both practices, while re-equipping them with highly different technical means, indicators, and charts. By designing and tinkering with electronics-based equipment, Earth has become quantifiable in a new metric, namely the isotopic signatures of geochemical flow, aligning and correlating the planet with a well-controlled experiment in the laboratory. In the end, isotope geochemistry has helped to entirely blur disciplinary categories—such that today “the boundaries between geophysics, geochemistry and geology are indistinct”25—while at the same time clarifying the start of the fundamental and radical changes that our planet started to undergo in the middle of the twentieth century.
This text is an edited extract from: Christoph Rosol, “Hauling Data. Anthropocene Analogues, Paleoceanography and Missing Paradigm Shifts,” Historical Social Research vol. 40, no. 2 (2015): pp. 37-66.
Christoph Rosol heads the research cluster Anthropocene Formations at the Max Planck Institute for the History of Science and works as a researcher and curator at Haus der Kulturen der Welt.
Please cite as: Rosol, C (2022) A Mid-Twentieth Century Start Date for Anthropocene Geology. In: Rosol C and Rispoli G (eds) Anthropogenic Markers: Stratigraphy and Context, Anthropocene Curriculum. Berlin: Max Planck Institute for the History of Science. DOI: 10.58049/4KWX-H491