Molecular Mobilization
How can an archaeology of the present address molecules as driving elements of the “Great Acceleration”? Benjamin Steininger, cultural theorist and also cultural practitioner, contends that the mobilization of combustion fuel molecules through the technical apparatus of catalytic chemistry has triggered a cascade of accelerations which lead to the fundamental transformation we now call the Anthropocene.
The Anthropocene, and specifically the “Great Acceleration,” is closely connected to the combustion of hydrocarbons in various types of engines on land, water, and in the air. It is no exaggeration to say that the type of history that humanity was facing and performing in the twentieth century would have been impossible without the internal combustion engine, without civilian and military aircraft, without cars, trucks, and tanks, and without global transport in containerships.
This type of mobility and mobilization was crucial both for human history in modernity, for the World Wars, world trade, global consumer capitalism, as well as for natural history in the Anthropocene. The traces and effects of engineered combustion are everywhere. They are present in the form of chemical materials as CO2, soot, or carbon black in the atmosphere, oceans, and soils, and as processes in all layers of human life and life in the biosphere. Thus understanding combustion as a chemical principle, the combustion engine as a technical device, and combustion fuels as specific energy carriers is crucial for any analysis of the Anthropocene.
This contribution tries to shed light on the specific technicality of combustion fuels in the twentieth century. It argues that behind the visible and prominent changes in the ways of moving humans, goods, and things in the twentieth century, a much less visible mobilization was set into motion—the mobilization of molecules. Petroleum and coal are products of biogeochemistry. However, these natural substances did not fuel history: only through the technical internal mobilization of natural and fossil molecules did the novel external mobilization of individuals, societies, and planetary layers arrive in the Anthropocene condition.
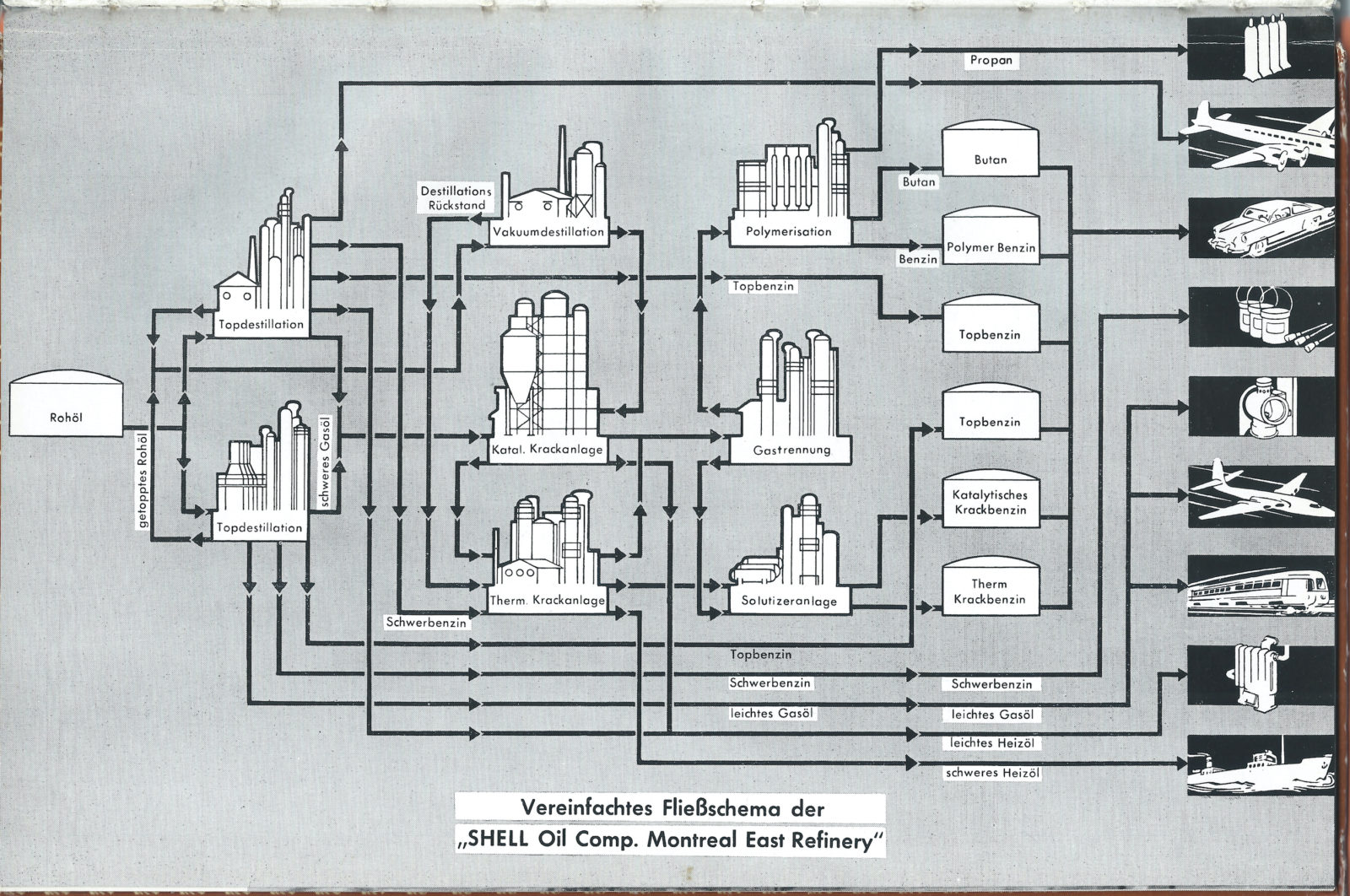
What still needs to be addressed is a large chemical-technical apparatus. A flow chart from 1955 published volume 5 of the Shell Bücherei, ABC des Erdöls (Shell Library, ABCs of Petroleum), titled Petroleum Cracking, provides an overview.1
“Molecular Mobilization,” a “simplified flowchart of the SHELL Oil company Montreal East Refinery,” showing the intermediate stage between crude oil (far left) and various applications (far right), including tap and vacuum distillation units [Tapdistillation and Vakuumdestillation], catalytic and thermal cracking units [Katal. Krackanlage/Therm. Krackanlage], gas separation [Gastrennung], and polymerization. Diagram from Gerhard H. Lehmann, ABC des Erdöls, Band 5, Heidelberg: Shell-Bücherei, 1955, p. 35 © all rights reserved original publisher
At the beginning is a tank full of (presumably unrefined) fossil liquid, which then enters a system of pipes and chemical reactors. At the end are exemplary products and process agents of modern life—airplanes, automobiles, boats, heaters, welding torches, paints and lacquers, just rockets are missing—but generally we see the world of the combustion engine.
The flow chart puts it clearly: Crude oil is only half of the story of mobility. The final product of seismic exploration, geology, and drilling techniques is the starting product of the refinery. So maybe more than the other half is chemical engineering. Since the 1920s, “science fashioned molecules” have been feeding engines.2 These molecules are manufactured from oil and coal at the refinery and chemical plant with the help of catalysts: substances that accelerate chemical reactions.
Thus, the fuels used to accelerate machines and bodies have themselves already undergone processes of internal acceleration—be it one of synthesis or the cracking of hydrocarbon molecules. Through the production of fuels, additives, lubricants—of every molecule that is needed to speed things up—the chemical industry reigns in this world of mobilizations. In order to go global, the Technosphere became molecular.
How can an archaeology of the present and of the Anthropocene address this chemical apparatus of molecular design and molecular mobilization? How can it reflect that already the molecules that drive all aspects of the “Great Acceleration” are human-made? How much of the particular industrial “biographies” and specific features of human-made fuels are present in the remnants of combustion?
In fact, there are archaeologies of fuels going on right now. One example is the attempts in Norway to retrieve engine fuels from World War II shipwrecks.3 There are dozens of shipwrecks of German and Allied origin. Their fuels are retrieved and analyzed to understand the impact of these substances on marine ecosystems. In fact, what many would think of just as an anonymous engine fuel at the filling station appears to be highly specific and very different. All fuels have a particular chemical “fingerprint,” similar to the way in which all crudes can be linked to particular regions or even wells by their particular chemical structure.
“Location of shipwrecks that were classified to have considerable pollution risk to the environment. The oil has been removed from the wrecks marked with a red circle. Map from NCA.” From: Liv-Guri Faksness, Dag Altin, Per Daling, and Kristin Rist Sørheim, “Report. Potential oil product leakages from World War II shipwrecks- Assessment of possible environmental risk,” SINTEF Materials and Chemistry. Environmental Technology, (September 1, 2016): p. 4. Map redesigned by Luis Melendrez Zehfuss
The most significant difference in the fuels in these shipwrecks is between “natural” British fuels from crude oil and “artificial” German fuels from coal—produced under well-known labels such as “Fischer Tropsch” or “I.G. Farben.” Interestingly, synthetic fuels are way more toxic than “natural fuels.” And of course, not only their chemical structures but also their industrial histories seem radically different.
Both historically and systematically, fuel production for combustion engines begins with the distillation of crude oil. Distillation separates the components of oil according to their boiling points, splitting off longer molecules from shorter ones and lighter molecules from heavier ones.
However, hydrocarbon molecules are not just fractionalized through distillation into natural substances of various qualities. It is the structures and qualities of the molecules themselves that are artificially changed by technologies such as “cracking” in which long molecule chains are broken apart; “reforming” in which they are rearranged; “cyclization” or “aromatization” in which they are closed into ring structures; “polymerization” in which they are joined with one another; and “hydration” in which they are combined with hydrogen.4
The most important tool in this kit of chemical building blocks is chemical catalysis. The flow chart features it prominently, even if it only cursorily describes it as as “Katal. Krackanlage” (“Catalytic Cracking Unit”). Catalysts are substances that accelerate chemical reactions without appearing in the reactions end product. The phenomenon was first described in the early nineteenth century as a kind of “contact effect” upon the discovery that an explosive reaction between hydrogen and oxygen can be caused, so it seemed, by the mere presence of platinum. In the 1820s, the chemist Johann Wolfgang Döbereiner, a friend of Johann Wolfgang von Goethe, conducted spectacular experiments on this marvel which provided the foundations for the commercial production of a lighter called Döbereiner’s lamp. Goethe himself thanked the inventor, “because your so fortuitously invented lighter is always by my side and the important discovery of such a powerful connection between two elements, the heaviest and the lightest, will evermore be useful to me in a wonderful way.”5
In 1835, Swedish chemist Jöns Jakob Berzelius coined the word “catalysis” for this type of reaction, drawing an analogy to “analysis.” But catalysis did not become the driving force of the chemical industry until the late nineteenth century. Working in the context of this new chemistry informed by the principles of thermodynamics, German chemist Wilhelm Ostwald coined the definition of catalysts as accelerators of reactions.6 In 1909, he was awarded the Nobel Prize in Chemistry for this insight.
Ostwald’s concept transformed a mysterious, uncanny force into a quantifiable event and a universal tool for chemical, industrial, and ultimately societal acceleration. The brilliance of his innovation was immediately clear. In a lecture held in 1901, Ostwald stated: “If you consider that the acceleration of reactions through catalysts occurs without expending energy, and is thus in a sense free of charge, and if you consider that in all technology, including chemical technology, time is money, then you see that the systematic application of catalysts promises the most profound transformations.”7 Indeed, in a matter of decades after Ostwald’s discovery, the materials produced with the help of catalysts—fertilizers, ammunition, fuels, pharmaceuticals, and solvents—triggered a “dromological”8 cascade of other forms of acceleration. The “Great Acceleration” has left no area of life untouched: mobility, war, population growth, the economy, and tourism, to name just a few. Described by authors like Ernst Jünger as a “total mobilization,”9 the mobilizations of automobiles, goods, people, entire economies, and even earth system processes10 that defined the twentieth century were made possible by the mobilization of molecules in chemical factories.
Catalysis is the core of this chemical mobilization, a fact that underscores how raw materials like coal and petroleum only make history when they are fed into entire systems composed of different substances and infrastructures. It is these systems that alchemize energy resources into, in the words of Jane Bennett, “vibrant matter.”11 Catalysts are vibrant matter in an almost technical and literal sense: they put other substances in motion by forming intermediates whose productivity in the chemical process derives from their very instability—“alternately being enwrapped and unwrapping themselves , being turned on and being turned off,” as Ostwald’s student, BASF laboratory leader and philosopher of catalysis, Alwin Mittasch, described it.12
Decisive innovations in the early years of the catalysis industry involved sulfuric acid and nitrates. But research on hydrocarbons is what enabled the development of synthetic catalysts that not only accelerate, but also control chemical reactions. This is what gave rise to the development of a chemistry working with building blocks as described above.13 Depending on whether iron, zinc oxide and chromium oxide, or zinc oxide, chromium oxide, and an alkali are used as catalysts, carbon monoxide and hydrogen can produce methane, methanol, or isobutyl alcohol. In other words, catalyzing hydrocarbons can produce molecules that consist of totally different quantities and arrangements of the basic building blocks carbon and hydrogen and, in some cases, building blocks like ethylene (C2H4) that are themselves synthetic. Catalysts can be seen as a second-order fuel. Moreover, some catalysts are themselves the product of the intentional, artificial rearranging of molecules: in many industrial applications, they are composed not only of natural substances but also of systematically-created synthetic compounds.
In the 1920s, one hundred years after Goethe’s praise of the “powerful connection between two elements, the heaviest and the lightest,” some other powerful connections were on the horizon: between coal chemistry and oil chemistry, and German and US companies. As Germany had almost no oil, but plenty of coal and a powerful chemical industry, in the 1920s attempts were made to convert solid coal into liquid fuel. One of the most difficult scientific and technical challenges for using catalysts to process coal was the almost inextricable presence of the chemical element sulfur. Sulphur can weaken the catalyst—it is what chemists speak of as a “catalyst poison.” To deal with this, BASF created sulfide catalysts that contain the “poisonous” element itself.14 They thus coopted sulfur into a kind of double agent, making it work for, instead of against, the catalysts. On the laboratory level, using sulfide catalysts seemed feasible, but it was difficult to scale up the process of coal liquification to an industrial level and impossible to compete with fuels from oil on the market. But in 1929, American financing stepped in to save the IG Farben’s undertaking—as the procedure seemed capable of not only liquifying coal, but converting inferior crude oils into the more desired fuels of gasoline and kerosene. In the words of Frank Howard, head of Standard Oil research, “It seemed clear that the German hydrogenation processes, and the new horizons they opened, were tremendously significant—perhaps more significant than any technical factor ever introduced into the oil industry up to this time.”15 Patents and stocks were shared, and cooperative projects and labs like Catalytic Research Associates (CRA)—a consortium that brought together German and American corporations—were established in Baton Rouge.
These intercontinental, inter-fossil alliances initiated a new phase of “Petromodernity”—just a few years before the partners found themselves on opposing sides of the Second World War. In 1942, the CRA led to the construction of the first catalytic fluid bed reactor, the “Catalytic Cracking Unit” in our 1955 flow chart. Though in theory not altered by the chemical reaction they accelerate, catalysts in a fluid bed reaction are regenerated in a circular process. The catalyst is present in the reactors as a fine powder that is pumped through the reactor and a regenerator alongside it. During the war, this method of cracking oil triumphed over liquefied coal, which could never be produced in sufficient quantities at German plants, located in places like Leuna, Pölitz, Wesseling, Blechhammer, and Scholven.
From: Liv-Guri Faksness, Dag Altin, Per Daling, Kristin Rist Sørheim, “Report. Potential oil product leakages from World War II shipwrecks,” p. 24. Chart redesigned by Luis Melendrez Zehfuss From: Liv-Guri Faksness, Dag Altin, Per Daling, Kristin Rist Sørheim: “Report. Potential oil product leakages from World War II shipwrecks,” p. 45. Graph redesigned by Luis Melendrez Zehfuss
All these historical processes and the mutual influence of German and US chemistry—with their scientific, industrial, and financial efforts and power—are concretely present in the fuels of the World War II shipwrecks in the Norwegian sea. In fact, the fuels differ more as chemical substances than as commercial. Furthermore, there would be no German synthetic diesel made from coal had the commercial power of the US oil industry not invested in it. The companies that during the war produced, on one side of the front “natural fuels” from oil and on the other side “synthetic fuel” from coal, before and after the war were friends.16
From an archaeological point of view, the Allied and German fuels appear with their chemical difference and their commercial vicinity as border objects. They mark the rise of a synthetic hydrocarbon chemistry, that included industrial and chemical dynamics from oil and coal. Just years after the war this integrated hydrocarbon chemistry would drive the Great Acceleration. An archaeology of combustion and fuels should have a closer look into these wrecks.
Conceptually, refining chemical raw materials brings with it a certain logic of substitutability. It is one of the goals of chemical science and industry to substitute rare or foreign natural materials with less rare and often domestic ones. Hydrocarbon chemistry which is able to put together almost every organic molecule with the almost binary and letter-like building blocks C and H, carbon and hydrogen, provides good examples of this logic. In particular, the success narratives of the German chemical industry are connected to the logic of substitution.17 In fact, the self-image of a nation without resources but with a strong chemical industry is still present today. The most paradigmatic colonial resource, sugar, was replaced, from the nineteenth century onward, by the industrial combination of domestic beet plants and fossil energy. Products in which coal was both used as a material and energy resource were Germany’s industrial substitutes for the dye indigo, for saltpeter used in the production of both ammunition and fertilizer, later rubber, and—in the case of coal-based liquid fuels—even crude oil. All these substitutes transformed global markets and material flows. And vice versa, the profits from these markets further improved the scientific tools of the chemical industry, an almost autocatalytic system, where the produced substances further accelerated the process.
As a tool of molecular mobilization, catalytic chemistry was what first disclosed the manifold potential uses of fossil resources. Today, roughly twenty to thirty percent of global GDP is dependent on the products of catalysis. And yet, despite the efficiency of catalytic processes, they are responsible for eighty percent of the energy used by the chemical industry and seventy-five percent of its greenhouse gas emissions. New catalysts and, perhaps more importantly, new process architectures are necessary in order to accomplish things like enabling multiple reactions to occur at the same time at low temperatures in a fashion similar to biochemical processes.
Catalysis researcher and Max Planck Institute director, Robert Schlögl, calls the goal here the “solar refinery.”18 He asserts that electricity generated from renewable sources—at some point occurring more efficiently than even natural photosynthesis—should be used for photocatalytic water splitting to sustainably produce hydrogen. This hydrogen could then be used to produce synthetic hydrocarbon molecules which can serve as fuels in existing infrastructures and burned in existing motors without carbon emissions. In this model, chemical technology saves the planet while at the same time producing economic values. As Schlögl puts it: hydrogen-producing chemistry will work as a “money printer.”19
The close connection of this type of chemical acceleration with the Great Acceleration sheds new light—but also casts new shadows—on well-known success narratives from the perspectives of the chemical industry. It was clear from the beginning that the commercial success of artificial materials would mean ruining markets of natural materials. BASF’s German profit with artificial indigo around 1900 was at the same time a loss for indigo farmers in British India.
Not only for the reason of industrial historical justice does the history of molecular mobilization need to be rethought and rewritten. Molecular manipulation and mobilization were and are some of the crucial technologies of the modern era and the Anthropocene—even if other technical regimes and revolutions such as nuclear technology or information technology would go on to have a much greater impact on the public imaginary. Commercial monopolies with all their geostrategic implications are also technical and chemical. The petrochemical industries are involved in almost every sphere of modern life through transport, fertilizers, plastics, and pharmaceuticals. In almost every activity of modern humans, substances are involved that pass through the above-mentioned petrochemical process landscapes where fossil substances are cracked into building blocks and rearranged. Preindustrial natural materials like indigo, saltpeter, wood, and whale oil carried with them a specific multitude of cultural and geographic narratives. Of course, there are not fewer cultural and geographic narratives in industrial products such as plastics, WD-40 lubricant, or industrial food, but they all share one specific industrial passage—down to the molecular building blocks and back into the desired products—that is part of all these new narratives. In that sense, a master narrative is woven into almost all Anthropocene matter.
Of course, there is no way back. Electric cars won’t be lubricated with natural substances such as olive oil or so-called spermaceti, a certain whale oil,20 and isolation materials for electronic devices won’t be picked from trees. Not less- but more-sophisticated “science-fashioned molecules” are needed as substitutes for the fossil base of modern life and technology. And the same chemistry that provided fossil materiality since the 1890s is now what must be mobilized to overcome this base. “Fossil reason,” the rationality specific to “hydrocarbon man,” is still the dominant available rationality of our present.21 All modern categories of science, individuality, and politics are undergirded with fossil fuels. Still, it is this very rationality that can and must be mobilized to also shape the materiality of the post-fossil-fuel era, when combustion will still take place but in a foreseeably climate-neutral way through the combustion of green hydrogen, synthetic hydrocarbons, and sustainable biomass (if not even at deeply ingrained cultural occasions such as campfires, ritual burnings, or candlelit dinners).
The archaeologic record from the shipwrecks in the Norwegian sea would not only tell the story of coal vs. oil or the wars of “hydrocarbon man,” but could also serve as a record for the prehistory of a new and different combustion era.
Benjamin Steininger is a cultural and media theorist, historian of science, and curator. For many years, his work has evolved around the complexities and contradictions of petromodernity.
Please cite as: Steininger, B (2022) Molecular Mobilization. In: Rosol C and Rispoli G (eds) Anthropogenic Markers: Stratigraphy and Context, Anthropocene Curriculum. Berlin: Max Planck Institute for the History of Science. DOI: 10.58049/0QXC-WZ17