Back to essay
How to read
?
This graph displays the temporal occurrences and relations of events within the essay. By scrolling through the text, key events are highlighted in the graph. Clicking on the graph expands it and arranges the events according to their position in the essay.
Do you want to help us with our research? Please, share your feedback with us!
The visualization is part of the VIDAN research project at UCLAB / FH Potsdam. More information.
The Anthropocene Signal Amidst the Noise
The Anthropocene Working Group is preparing the case for the definition of a new stratigraphic Epoch: this requires the demonstration of an objective change in properties of a geological archive over time. As each property being examined for this distinction can be considered as a time series, we encounter a classic scientific problem of distinguishing signal from noise. We need to understand the reasons why a signal of change should exist in order to know how to look for it—for example, in what chemical form would a new pollutant emerge? We also need a theory (a model) of the noise—over time, how large are the natural background fluctuations, and what are their properties? A familiar example of this challenge is the tricky problem of distinguishing long-term climate change from the background of weather events. In this essay we examine these issues with respect to several chemical signals of environmental change which reflect global (anthro-)(bio-)geochemical cycles. To begin, we see what can be learned from the extensive measurements of sea-level change: an instructive example of a high-quality source of data with considerable noise in terms of geographic variation.
Variations across time and space: Sea-level change
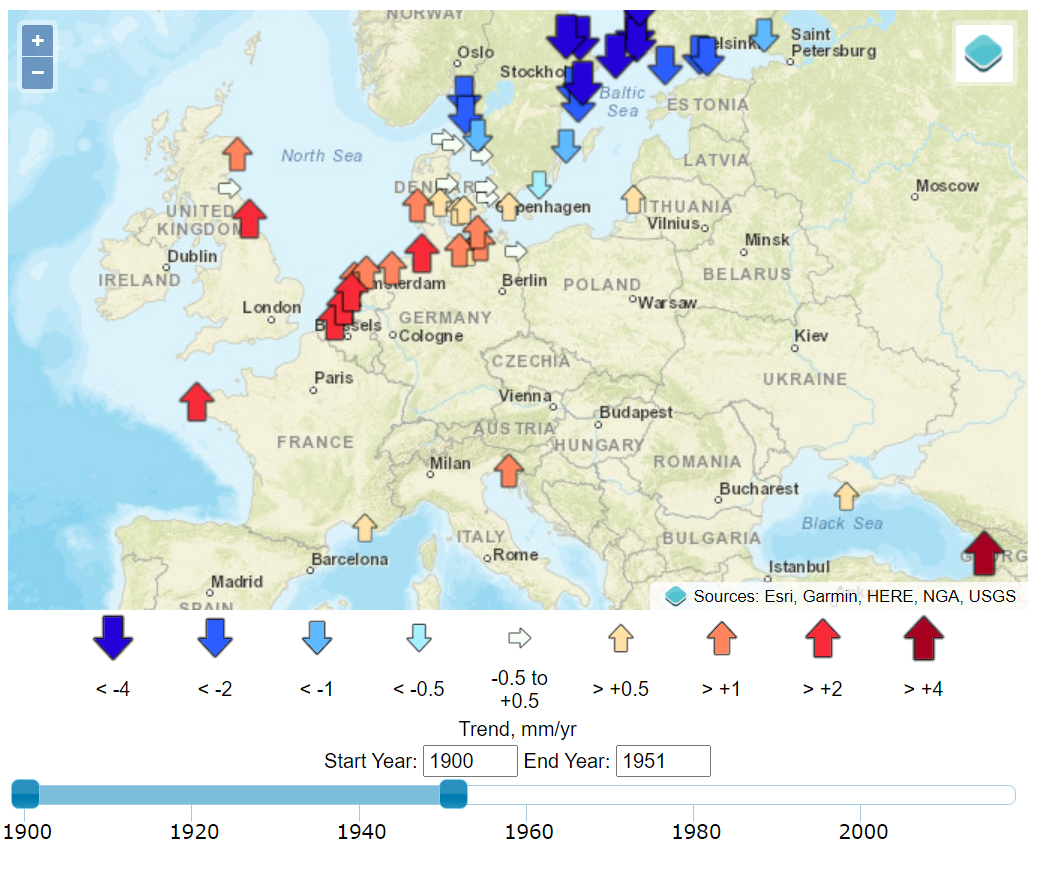
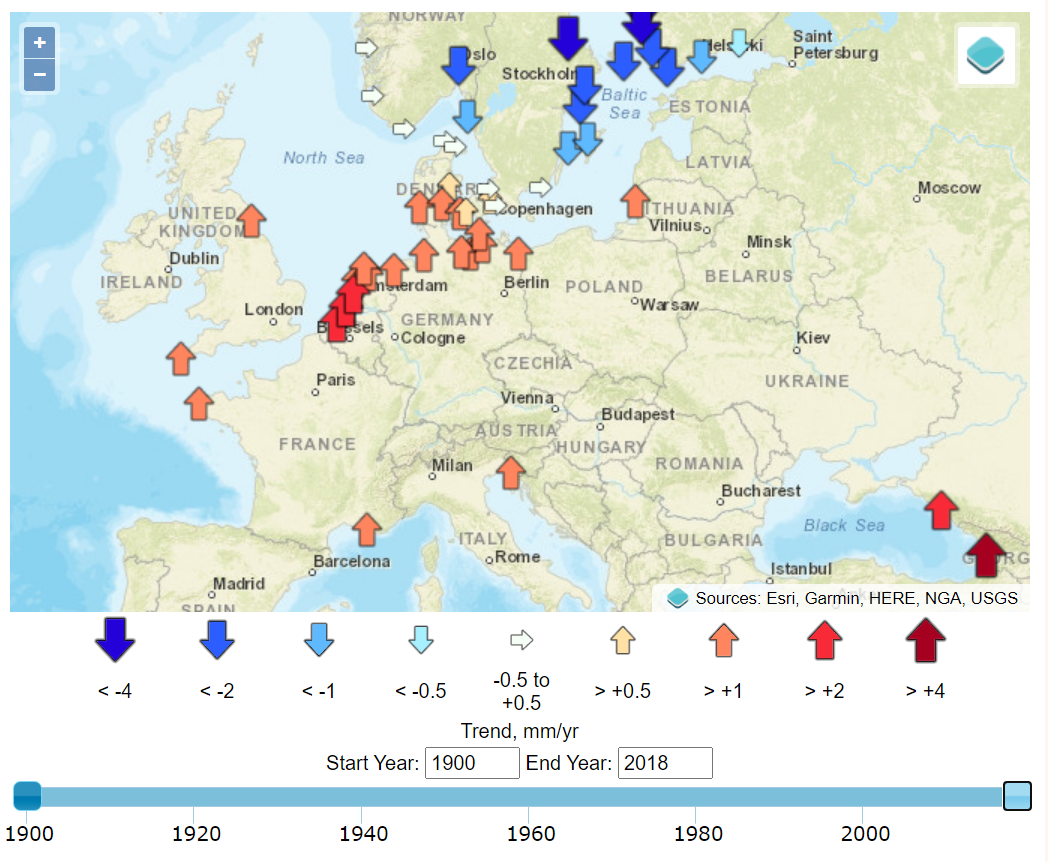
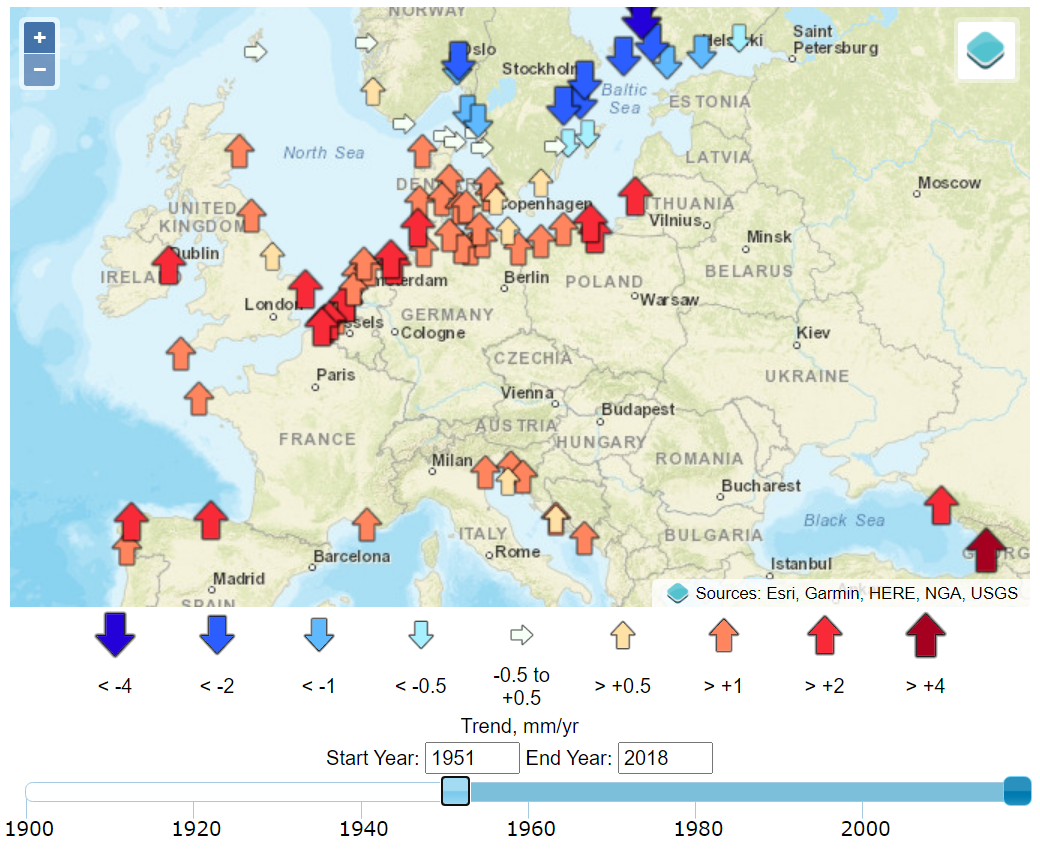
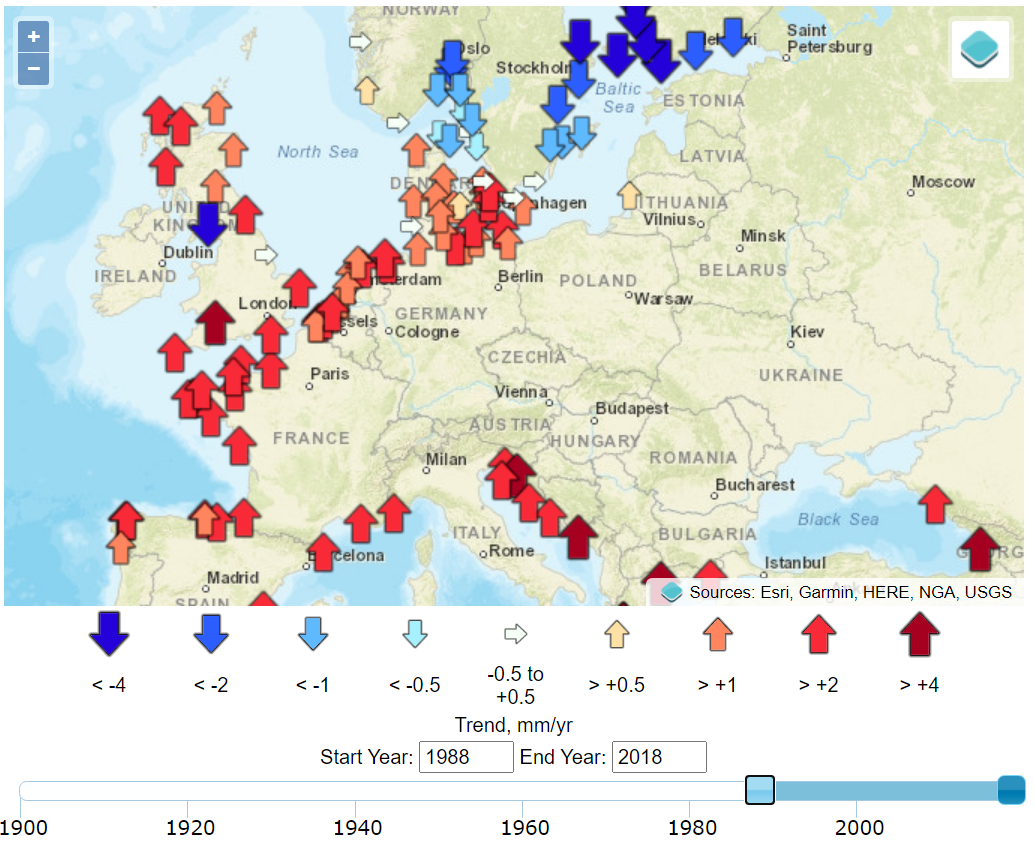
Today, As these coastal environments are extensively reclaimed for agriculture, urbanization, and industry, sea-level rise is an existential threat to many communities. began in Amsterdam in 1682 and when in the 1930s. Sea level at a given location represents the mean height of the water surface relative to the underlying geological substrate. Establishing this mean requires time-averaging myriad fluctuations related to climatic and geophysical factors.1 Tides, for example, display a range of variabilities on longer timescales than the familiar diurnal variation, and year-to-year variations in storminess can be significant sources of noise. While tide gauges provide data that is highly precise and accurate in relation to time and space, their signal is local, and as we examine below, their noise is related to geographic variations.
To to the late twentieth century when , we must use geological data. Salt marsh sediments contain microfossils whose narrow height distribution within the tidal range is delimited by modern studies. The sediments can be dated by radiometric analyses (14C, 210Pb) and the use of stratigraphic markers of pollutants, including metals, rare isotopes from atmospheric nuclear test measurements, and human-made pollutants. The resulting records of a in the twentieth century and a vertical resolution within ±5 cm.2
The primary anthropogenic disturbance to the pre-existing long-term trends in sea level is the emission of greenhouse gases. Since the mid-twentieth century, .3 Consequently, , , , : accordingly, .4 Sea-level change is one of the most important consequences of the Quaternary (last 2.6 million years) climate variability, as the sea level decreases during glacial intervals and increases during interglacial phases. Comparing the tide-gauge and geological records shows that the .5
We now know the direct causes of this change precisely because of the advent of satellite monitoring complemented by accurate surface devices (such as autonomous floats) in the ocean. began with the , and were later enhanced by the GRACE satellite mission, which uses measurements of gravity to understand ice mass loss from ice sheets and glaciers. since the early 1990s while .6
The timing of the measured sea-level inflections matches, with a delay of about a decade, the stepped changes in atmospheric carbon dioxide concentrations.7 Additional noise in the time domain of the calculated global mean trends of sea level is observed to coincide with volcanic eruptions (negative deviations) and the 2016 Super El Niño and 2011 La Niña events (positive and negative deviations, respectively), as can be seen in the Global Mean Sea Level chart provided by the University of Colorado. Other processes, such as ocean dynamics, tectonics, or glacial isostatic adjustment are spatially variable and cause sea-level rise to vary (usually very slowly) in rate and magnitude between different regions. Local and regional changes in these climatic and geophysical factors produce significant deviations from a global average rate of sea-level change.8 These dynamics are well illustrated by the interactive graphics at the Permanent Service for Mean Sea Level (PSMSL) based at the National Oceanography Centre in Liverpool.
since the beginning of the twentieth century. , primarily associated with Using data from recent decades, By contrast,
(2019) predict an , (see below). The spread within the forecasts reflects the uncertainties that exist about the future behavior of the Antarctic and Greenland ice sheets.
Climate signals in ice cores: Oxygen and hydrogen isotopes
Certain elements and their isotopes (atoms of the same element differing in mass) can be used as geochemical tracers of anthropogenic impact. Our has come from the geochemistry of oxygen isotopes, originating from the in the late 1940s. Workers have traditionally used mass spectrometry to make extremely precise measurements of the ratio of abundance of a rare isotope such as 18O (spoken as oxygen-18) to a common isotope such as 16O on a gas produced in the laboratory from the material (e.g. water or calcium carbonate). The ratios are normally expressed as the delta value, e.g. δ18O, a number in parts per thousand (‰) expressing the relative abundance of 18O to 16O. Lower delta values mean both less 18O as seen in water vapor compared with its source water, and in a progressively greater lowering of 18O in rain and snow from cooling air masses. Hence ice sheets, primarily formed from evaporation from sea water, have very low delta values which, during an ice age, lead to a higher δ18O value of the remaining seawater. The larger the mass of ice, the larger this ice-volume effect. in 1967. As a result, , and we know that in glacial stages, the global temperature averaged 4–6°C below present values but was 9–11°C below present values in Antarctica.
Although a (small) oxygen isotope (δ18O) signal does mark the base of our current interglacial period (the Holocene) in a Greenland ice core, the subsequent variations are more subtle and below the 0.5–1°C sensitivity of the oxygen isotope proxy. Consequently, an assemblage of temperature proxies is used, rather than just oxygen isotopes, to establish Holocene temperature archives. When it comes to the mid-twentieth century , there is no discrete (δ18O) signal in oxygen isotope archives, even in the relatively sensitive Antarctic records. In fact, the gradual increase in the rate of temperature change makes it difficult to define an Anthropocene boundary on the basis of any geochemical proxy for temperature. This problem is also the basis for the (also known as the “boiling frog syndrome” of inattentiveness to gradual risk increases).
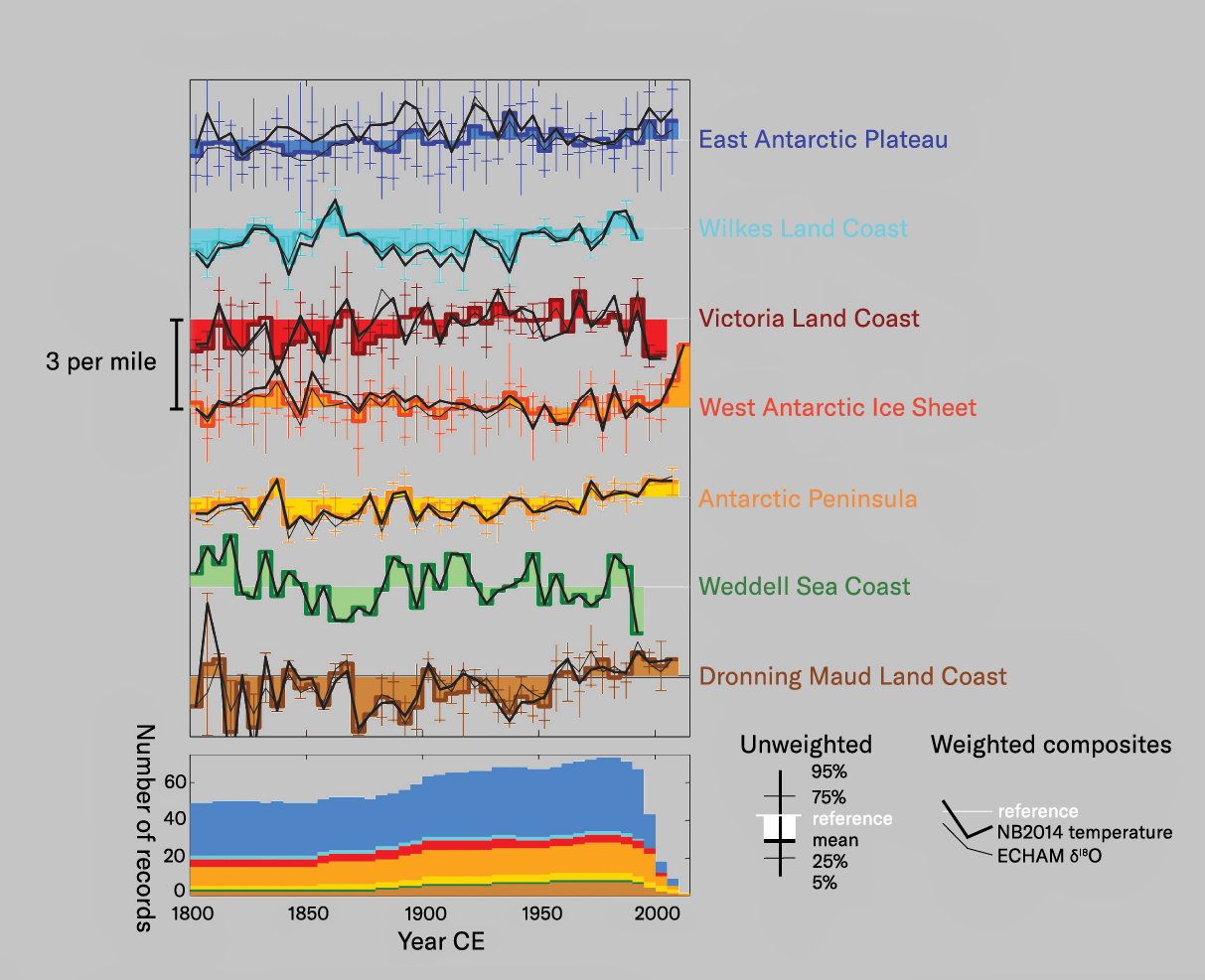
The in recent decades does, however, lead to . Hydrogen isotopes show covariation with oxygen and with temperature, so measuring both elements provide a more robust measure of past temperatures. To avoid local effects, the most reliable signals are reached through the combination of multiple ice-core records from a given site and/or region, and through the site-specific calibration of the relationships between water-stable isotopes and temperature ,9 which has been carried out under an international project identifying several discrete regions of Antarctica that exhibit somewhat different climate signals (see below). Temperature reconstructions based on the isotopic data showed warming in recent years in three of these regions. In each case the began around 1920 and continued to the latest available measurements (ca. 2005) with rates of warming between 1 and 2°C/100 years. These increases followed that lasted over the previous 2,000 years. However, relative to the for the past 2,000 years, only the rise in temperature of the Antarctic Peninsula is unusual. It seems reasonable to assume that because of its more northerly aspect, the Peninsula is subject to a slightly different climatic regime than most of the continent, and one that is closely connected to the global warming identified elsewhere on the planet. Close inspection of the isotopic temperature signal for the Peninsula suggests after about 1970 than between 1920 and 1970, much as is found in the global surface temperature signal. It seems likely, then, that the Peninsula temperature signal reflects after 1900, followed by after 1970, as on the rest of the globe.
Regional composites of stable oxygen isotope values, in each case calculated using two different methods. All anomalies are expressed relative to the 1960–1990 CE interval. Diagram redesigned from Stenni et al. 2017 (footnote 9) by Luis Melendrez Zehfuss
The carbon cycle and sulfur in the atmosphere
A primary driver of global warming is the burning of fossil fuels which have created a first-order change in the carbon cycle. Fluxes of carbon between reservoirs have increased, and carbon, mainly as carbon dioxide, is increasingly stored in the atmosphere. This newly released carbon has a distinct fingerprint in terms of the ratio of the stable isotopes of carbon (13C/12C) as determined from mass spectrometry. Expressed in terms of the delta number (δ13C), the rare 13C is much less abundant in the biosphere (δ13C around -25‰), and thus in fossil fuels that stem from organic material, than in the pre-industrial atmosphere (-6.5‰). Hence atmospheric CO2 trapped in ice cores shows an inverse relationship to δ13C, known as the around 1965 CE. This signal in ice cores shows little noise because it reflects the composition of a well-mixed atmosphere. It therefore compares in quality with that of radioactive species such as radiocarbon and plutonium released through atmospheric nuclear tests. Carbon isotopes in other archives typically show a less clear-cut relationship because of the complexities of biochemical cycling. For example, this signal is masked altogether in cave carbonates (speleothems) because of large fractionations in carbon isotopes in cave systems. Trees provide an interesting intermediate example where, although the carbon fixed is derived directly from the atmosphere, seasonal to decadal changes in tree physiology add noise to the Suess effect. However, in the dataset shown below,10 a moving average of the around 1960 CE. Marine records, such as from corals, also demonstrate a clear signal.
Speleothem core from the Ernesto Cave GSSP site in Italy. Photo by Renza Miorandi, © all rights reserved Andrea Borsato
A contrasting example is provided by sulfur in the atmosphere. It is released naturally from volcanic eruptions, but emissions increased enormously because of industrial processes, particularly the burning of sulfurous coal. Because this sulfur is in the form of microscopic aerosol particles, the abundance of sulfur in geological archives and its stable isotope composition show regional variations.11 For example, These records rely on micro-scale analytical techniques at specialized facilities, such as ion probes or the fluorescence produced by X-rays in huge synchrotron facilities. Both are anthrobiogeochemical records since the O and S isotopes in sulphate in rainfall become fractionated when stored in soil humic substances. This pollution indicator is also seen as an anthrogeochemical (i.e. non-biological) signal in Greenland ice, but is absent in Antarctic ice cores.
δ13C variability from Loader et al. 2013 (see footnote 10) for the period 1500–2008 CE measured in tree-ring cellulose for a composite tree ring stable isotope chronology developed using Pinus sylvestris trees from northern Fennoscandia. Fine line represents annually-resolved δ13C variability, thick solid line presents the annual data smoothed with a centrally-weighted 51-year moving average. Dashed line represents the mean δ13C value for the “pre-industrial” period 1500–1799 CE. Mean annual replication for the record is >13 trees. Analytical precision = 0.12 per mil. Graph redesigned by Luis Melendrez Zehfuss
The nitrogen cascade
The modern nitrogen cycle is a typical anthrobiogeochemical cycle because human activities have led to substantial changes in natural processes governing nitrogen fixation, its bioavailability, and its overall fate in the environment. The since the beginning of the twentieth century constitute one of the most important sources of anthropogenic perturbations of the nitrogen cycle, along with the burning of fossil fuels and symbiotic nitrogen fixation by bacteria hosted by cultivated plants.
An excess of nitrogen causes a negative impact on all environmental compartments. In surface waters it brings about eutrophication, leads to a deficit in oxygen (hypoxia) in the sea, and a decreased input of dissolved silica in soils. Excessive amounts of nitrogen cause acidification and decreased biodiversity. It also diminishes the quality of air through the formation of particulate matter and ozone. The above examples of complex environmental impacts are often referred to as a “nitrogen cascade.”
Nitrogen has two stable isotopes, 14N and 15N, with 14N making up 99.63 percent of all nitrogen occurring in nature. Anthropogenic sources produce a change of nitrogen stable isotope ratios in different environmental archives. This change may potentially be used as a signal of the Anthropocene. Sample analysis is again by mass spectrometry with preparation techniques tailored to create gas for analysis from materials such as organic matter or ice. The increase in concentrations of nitrogen compounds in the environment, especially from fossil fuel burning, is usually accompanied by a decrease in δ15N values. For example, the Similar patterns of δ15N values were found in analyses of total nitrogen in organic carbon from dated lake sediments collected from 25 remote and nutrient-poor lakes in the Northern Hemisphere. In many environmental archives, the rate of decline in the δ15N value has accelerated and become pronounced, but there are also during recent decades. In coral skeletons from the Pearl River estuary in China, for example, were recorded in the skeletons dated from the mid-Holocene to the 1980s, followed by an increase to >13‰ during 1987–1993 CE and later a (below).12 Nitrification (oxidation of NH4+ from sewage to nitrites and nitrates) and subsequent denitrification (reduction of nitrates to N2O and N2) caused an increase in the δ15N values because the products of denitrification were enriched in the lighter N isotope, whereas the remaining inorganic N species were enriched in 15N. It is fortunate that despite the complexities of the biogeochemical cycling of different nitrogen species, both the nitrogen isotope records of nitrate and the organic carbon fraction reflect environmental change. The observed trends in the δ15N values of different environmental archives reflect a combination of natural- and human-driven processes, thus confirming the usefulness of stable nitrogen isotopes for our understanding of anthrobiogeochemical nitrogen cycles.
Examples of δ15N profiles in different environmental archives; Greenland Summit ice core (blue); coral skeleton (orange); alpine lake Beauty (green). Data compiled from Holtgrieve et al. 2011 (ice core, lake sediment) and Duprey et al. 2020 (coral skeleton). See footnote 12. Graph redesigned by Luis Melendrez Zehfuss
The legacy of lead
Trace metals are integral parts of natural element cycles and, before human interference, came mainly from geo-/lithogenic sources via the weathering of rocks. We focus here on lead (Pb), as it is relatively common in the Earth’s crust (average 14 ppm) compared to other trace metals, has been widely mined or formed a by-product of mining since prehistoric times, and is identified as a highly toxic element, making the monitoring of Pb contamination as well as searches for its provenance a critical issue in geological, environmental, and public research. Beginning as early as thousands of years BCE, the : initially lead was merely a by-product, and only later was mined directly.
Many different techniques are used to determine total Pb concentrations in samples, and the analytical precision of these techniques is far less than the sample-to-sample variation. Ratios of stable isotopes such as 206Pb, 207Pb, and 208Pb analyzed by different types of mass spectrometry can be used to distinguish the sources of contamination by lead in the environment, e.g. in assessing the relative contributions from mining against natural background values.13 Natural background fluctuations vary across orders of magnitude, driven by local high-lead sources like ores, variabilities in input from weathering, and sediment dilution. Remote archives such as ice sheets largely show inputs of natural dust particles, contaminated by anthropogenic emissions such as those from lead in petrol. Because ice sheets are remote, background concentrations of Pb in Arctic and Antarctic ice cores range from a few to a few tens of parts-per thousand, whereas in other archives, such as lakes and bogs, natural background concentrations are mainly around tens of parts-per million.14
The can be identified in Northern Hemisphere ice cores from Greenland and Arctic Canada, and are linked to some 3,000 years ago (see below). .15
Later, silver, and thus also lead, mining and usage increased substantially during Roman times, which lead to significant human health problems. This usage is recorded in archives by a widespread lead peak, more pronounced than the earlier one, and is recognized in ice cores, estuaries, bogs, and remote lakes of the Northern Hemisphere; a Spanish source for the lead was revealed by isotope studies.16
Global lead production and usage only began to rise again with silver production in medieval times. This was followed by an even larger rise due to silver mining and production in the New World, with abundant production and use punctuated by plagues and wars. The around 1850–1890 CE that was much larger than the Roman peak. The beginning around the 1850s onward formed the was reached during the mid-twentieth century, when Although it was not as pronounced as are other chemical markers around the 1950s, Due to the eventual recognition of its toxic behaviour, from the 1970s onwards. The recent widespread in the 1920s, , and , arriving at today’s .17
Holocene Pb concentration and 206Pb/207Pb ratios from the Arctic ice core section at Devon Island, Canada (D1999 core, Devon Island Ice Cap, Nunavut). Grey rectangles mark levels of anthropogenic lead input around 3000 BP, 2000 BP, medieval times and the Industrial Revolution and Great Acceleration lead peak. Graph redesigned from Wagreich and Draganits 2018 (footnote 15) by Luis Melendrez Zehfuss
The Anthropocene signal amidst the noise
The large amounts of industrially-produced pollutants and emissions that have been introduced, over decades and centuries, into air, soil, and water have caused considerable changes to natural phenomena, such as the cycling of elements and changes in sea level. However, the Anthropocene signals lie amidst noise which arises for several reasons. One is the issue in making measurements with sufficient precision (easy for tide gauges, but tricky for some rare isotopes). Another is the geographic variability of many parameters. A third is the presence of natural variability over time. Despite these problems, many different types of anthropogenic signals have become increasingly prominent and isolatable since the middle of the twentieth century.
Ian Fairchild is Emeritus Professor at the School of Geography, Earth and Environmental Sciences at the University of Birmingham.
Alejandro Cearreta is Professor of Micropaleontology at the Universidad de Pais Vasco UPV/EHU, Spain, Head of the Geology Department and Director of the postgraduate program in Quaternary: Environmental Changes and Human Fingerprint.
Colin Summerhayes is Emeritus Associate at the Scott Polar Research Institute of the Geography department at the University of Cambridge.
Agnieszka Gałuszka is Full Professor at the Institute of Chemistry, of the Jan Kochanowski University in Kielce. She is an expert in geochemistry and biogeochemistry.
Michael Wagreich is Full Professor of Geology at the Department of Geology, Faculty of Earth Sciences, Geography and Astronomy, of the University of Vienna.
Cover art by Protey Temen, © All rights reserved Protey Temen