Closed Chemical Cycles
A Future Marker for the Anthropocene
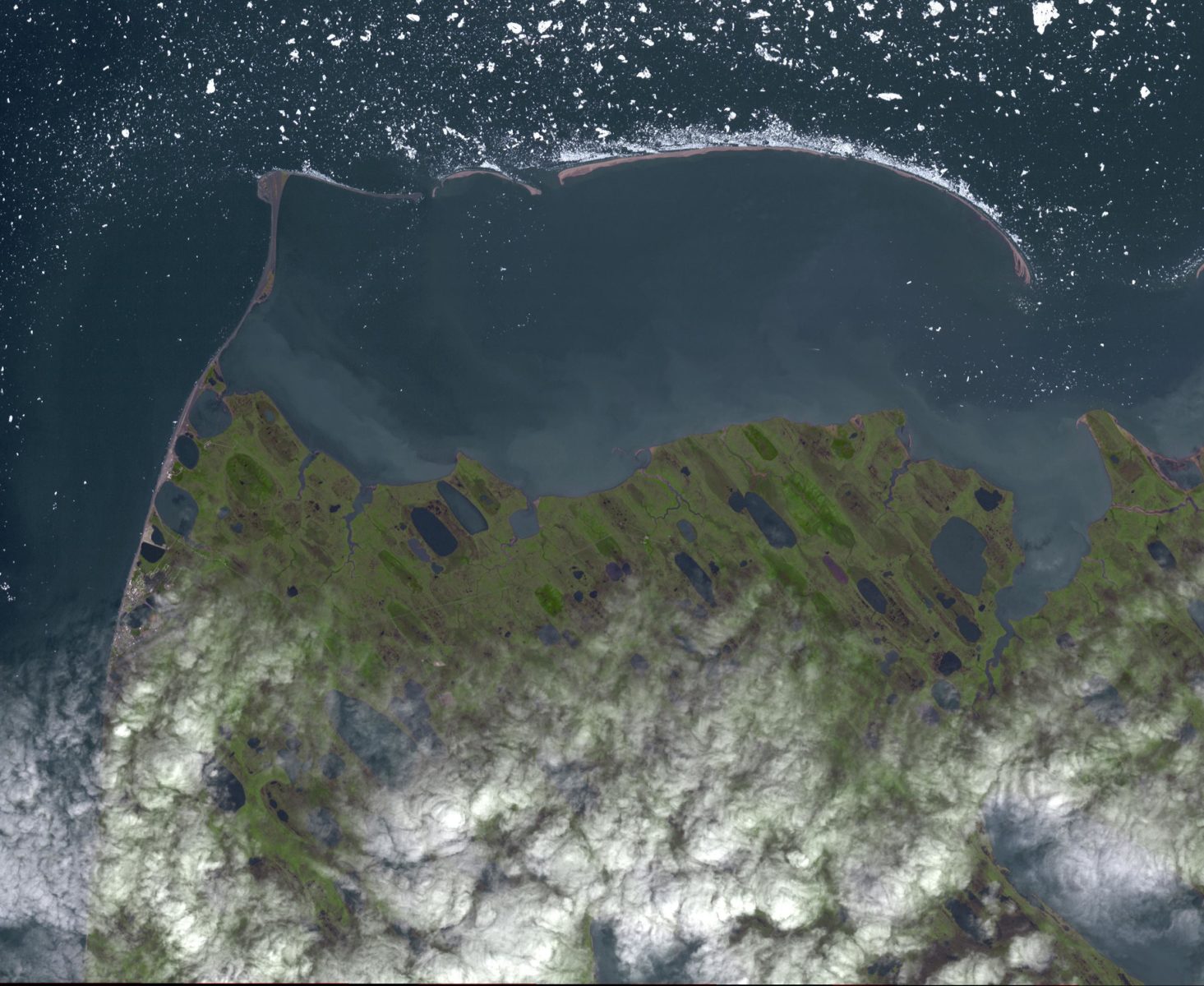
Considering the hitherto short duration of the Anthropocene, it’s possible that the most suitable golden spike for geologically marking the epoch’s beginning does not yet exist. Physicist and historian Benjamin Johnson argues that if Earth-human interaction is to endure and eventually merit recognition as a geological age, one necessary development is for humans to close the biogeochemical cycles they have fundamentally perturbed. Such an intentional closing would be linked to human action and could be precisely marked in time, presenting a clear sign of a stabilizing Anthropocene. Born and raised in Alaska, Johnson accompanies his contribution with images from the state’s northernmost point, Point Barrow, or Nuvuk, where the changing carbon concentration in the atmosphere has been tracked since the 1970s. Nuvuk acts as a reminder that even the most remote regions of the globe are impacted by anthropogenic action.

From the benzene ring to open (anthro)biogechemical cycles
In 1825, the physicist and chemist Michael Faraday published his observations on the decomposition of oil by heat and described one of the substances he found as “bi-carburet of hydrogen.”1 He claimed it was a compound composed of two parts carbon and one part hydrogen. As the natural philosophers of the nineteenth century made headway in the labyrinthine field of organic chemistry (essentially the chemistry of compounds containing carbon-hydrogen bonds like benzene and methane), they identified other substances with the same ratio of carbon-to-hydrogen as found by Faraday, but displaying different properties. The results were baffling (we now know that even simple molecules containing only carbon and hydrogen can combine in any number of ways as isomers), and the complexity of the issue quickly overwhelmed the scientific methods available at the time (that is, prior to 1900).
But this lack of knowledge about isomers was not the only problem. Faraday also didn’t know that pure hydrogen exists as a molecule of two atoms. His assumed weight for hydrogen, then, was off by a factor of two, meaning that his new compound actually consisted of equal proportions of carbon and hydrogen (thus “carburet of hydrogen”). Throughout the next half century, painstaking studies revealed the makeup of many organic molecules, including the substance first described by Faraday. In 1865, the organic chemist Friedrich August Kekulé succeeded in predicting the form of “bi-carburet of hydrogen”: a ring of six carbon and six hydrogen atoms. Today, we refer to it as the benzene ring—a structure that has played a conspicuous role in humans’ interference with biogeochemical cycles. In fact, the composition of this molecule alone—C6H6—shows how the carbon and hydrogen cycles are inherently and inextricably linked.
Before the chemical-industrial developments that led to open planetary cycles could play out, however, the understanding of chemical composition needed to be complemented by an accurate description of chemical behavior during reactions. Not only did the complexity and diversity of organic bodies make them difficult to classify, but even the most sophisticated mechanical theories of the day were unable to explain their chemical dynamics. A holistic approach was needed, one that could smooth out the details of individual bodies and sum up everything with one overarching concept. By 1865, this notion existed in the form of thermodynamics, based on the concepts of energy and entropy. At this point, it could successfully describe the behavior of gases, and over the next four decades, the concept was extended to chemical reactions in solution, giving birth to the hybrid field of physical chemistry.
During the same period, the benzene ring was manipulated to produce aniline: a benzene ring in which one hydrogen atom is replaced by the amino group, NH2–. This organic molecule would go on to form the basis of the German chemical and dye industry, an enterprise that built up a vast industrial infrastructure for the production of synthetic chemicals along with heavy financial clout. The knowledge from physical chemistry and the means provided by the chemical industry established an entirely new playing field for chemical manipulation. Within this context, scientists like Henry-Louis Le Chatelier, Wilhelm Ostwald, Fritz Haber, and Walther Nernst were able to combine the existing knowledge into new forms and dimensions of chemical production. For example, they made crucial contributions to understanding ammonia synthesis from the elements, a process that would transform the fixed nitrogen industry and provide the foundation for today’s high-pressure catalytic industry. These technologies have, respectively, allowed us to solve limitations on fertilizer production and refine oil into fuels to transport energy wherever we might need it.
A common lament in this context is, of course, that these successes of science and technology have also resulted in unintended consequences that place humanity, once again, in the position of having to solve overwhelming—perhaps existential—problems. By harnessing the power of chemical manipulation, we have opened new and extensive input channels into the biogeochemical cycles (carbon, nitrogen, phosphorous, potassium, rare earths, etc.) that constantly move through the bio- and lithospheres. As per the nature of the linear economy, the result has been a buildup of substances that become detrimental at certain concentrations. Carbon dioxide and methane strengthen the greenhouse effect; nitrogen runoff causes eutrophication (the mineralization of lakes and other bodies of water).
Some scientists have proposed this opening and interlinking of the biogeochemical cycles as a geological marker for the Anthropocene. However, considering the hitherto short duration of the Anthropocene as a geological age, it is possible that the most suitable golden spike is not identifiable because it does not yet exist. If the Earth-human interaction is to endure, the array of potential markers will certainly increase. One development of a “successful” Anthropocene will be that humans eliminate or alter the inputs they have contributed to the chemical cycles and establish methods of driving these cycles in a more closed way.
The word “successful”—though perhaps snarky—alludes to the fact that if we do not close-off human-produced inputs into the biogeochemical cycles, the Anthropocene will surely be too short to warrant consideration as a geological age (at which point any remaining humans will have more serious problems than selecting a geological marker). Closed chemical cycles are the foundation of a circular, or O-, economy and can help us gauge the trajectory of (sustainable) consumption in the future. They may also be key to reversing the Great Metabolic Anomaly (Große Stoffwechselanomolie).2 Furthermore, closed chemical cycles could control and reduce the accelerating exchange of energy and materials between humans and our environment—at least when considering net values. Any remaining unsustainable consumption would be negligible if it were returned to levels low in comparison to the natural growth periods of the biosphere.
How to close an anthrobiogeochemical cycle
Strategies for closing the biogeochemical cycles have been developed. In fact, some procedures are already in place and functioning at various scales. And, as further explored below, they also mark a change in our approach to energy conversion.
Admittedly, closing all the biogeochemical cycles—especially of phosphorous, nitrogen, and other agricultural elements—is a formidable task demanding an array of chemical and technical solutions on both land and sea, some of which have not yet been developed. Possible strategies include improved wastewater treatment and mariculture.3 Carbon (in the form of CO2), however, can serve as a succinct and crucial example, especially considering its role in the greenhouse effect. Focusing on this element illuminates several strategies already capable of contributing to closing chemical cycles. The most important is a society-wide reduction in consumption, which requires education and self-discipline but, by itself, will not get us to net-zero carbon emissions. Two other approaches are carbon capture and storage (CCS) and carbon capture and use (CCU). With current technology, CCS is an option. However, storing carbon as carbonate in bedrock, for example, does not close the “geo-” part of a biogeochemical cycle. CCU, on the other hand, while not yet technologically mature, offers the possibility of realistically establishing a net-zero carbon cycle in conjunction with the decarbonization of suitable sectors. It is also a powerful illustrative tool for the concept of a circular economy.
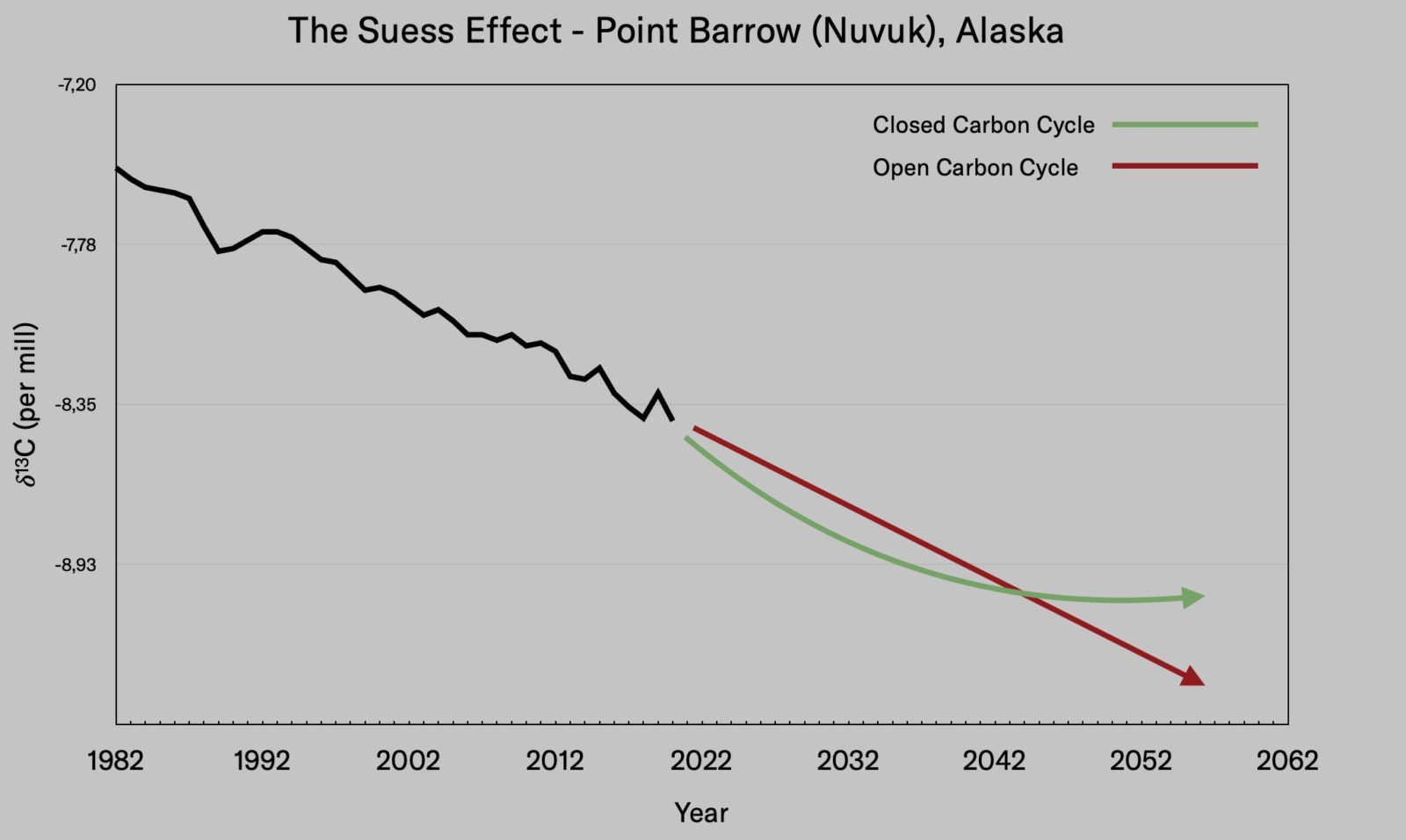
The Suess effect as measured at Point Barrow or Nuvuk, Alaska, with future projections based on closed (green) or open (red) carbon cycle scenarios. Data: C.D. Keeling, et al., Exchanges of atmospheric CO2 and 13CO2 with the terrestrial biosphere and oceans from 1978 to 2000. Global aspects, SIO Reference Series, No. 01–06, Scripps Institution of Oceanography, San Diego, CA (2001). Graph courtesy Benjamin Johnson; redesigned by Luis Melendrez Zehfuss
To be sure, however, we would be well advised to take all options—CCS and CCU and any others—seriously as climate-mitigation tools.4 At this point, we do not have the luxury of being exclusive.
The viability of CCU is an option born of the ability to harvest power from inexhaustible sources, such as the sun and wind. Historically, while nature has always interconverted forms of energy as the need arises with little regard for efficiency, humans tend to be more purposeful in their choice of energy conversion. The most basic is the conversion of stored chemical energy in food into heat, electrical, chemical, and kinetic energy in the body (although these processes are as much nature’s doing as our own). The act of throwing, for instance, imparts the kinetic energy of our arm to another object, and a bow and arrow impart kinetic energy to an object, which arises from the potential energy stored in a string after work is performed by our muscles. Another energy source for early humans, fire, is the single-step conversion of chemical energy into heat. Later examples include the waterwheel and windmill (kinetic energy → kinetic energy) and the cannon and steam engine (chemical energy → heat → kinetic energy). These forms of energy conversion are mainly without direction (waterwheel: kinetic → kinetic) or unidirectional (cannon: chemical → heat → kinetic). The generation of electricity in the nineteenth century allowed for some variation of this linear pattern. The original strategy behind electrical generation was to transport the ability to do work (waterwheel, steam engine) to a region where the kinetic energy of water or chemical potential energy of coal was not available.5 As opposed to the non- or unidirectional conversions of earlier forms of energy, electrical energy could be converted back into kinetic energy to perform work (as opposed to using electricity as the end product for, say, a light bulb). The conversion chain was, thus: kinetic energy → electricity → kinetic energy. Or, in a more detailed form, using coal: chemical potential energy → heat → kinetic energy → electricity → kinetic energy. However, the bottom line with the choice for converting energy to electricity revolved around economic considerations. That is, the central aim behind electricity’s use was maximum economic efficiency.
Efficiency remained a dominant theme in Western life throughout the twentieth century. As electrification expanded, the sources of electricity remained largely based on nineteenth-century technology: hydropower or steam produced by the burning of fossil fuels drove an induction coil to generate electricity. The second half of the twentieth century saw the introduction of nuclear power, but it did not change the basic mechanism of electrical generation. Only the old source of heat—the oxidation of carbon—was replaced, by fissioning atomic nuclei in a controlled chain reaction. The last quarter of the century saw further changes, though they made up only a tiny fraction of the entire energy regime. Wind farms, for example, did do away with the conversion step to heat but still depended on induction. In all these processes, kinetic energy (either natural or human-harnessed) is used to make electricity, which is sent to an end user for a variety of applications. At the heart of this process lies a consumption chain that remains linear.
Solar cells, however, represent a significantly different approach. Here, the power of the sun is harvested and converted into electricity in a single, integrated step. A solar, or photovoltaic (PV), cell is essentially a large surface diode, commonly made of silicon, which contains a built-in electric field. When a photon excites a bound electron into a free state within this field, the electron is swept to one side of the solar cell. This results in a separation of charge, which creates a potential difference between the two poles of the cell. If an external electric circuit is connected, the voltage produces an electric current that can perform work.
How does energy production with solar cells and wind turbines lead us toward a circular mentality with respect to biogeochemical cycles needed for a sustainable Anthropocene?
The key is that both are emissions-free, inexhaustible energy sources driven by the sun. In the case of PV cells, it’s as electromagnetic energy directly from the sun, and in the case of wind turbines, it’s kinetic energy imparted to the atmosphere via temperature and pressure differentials. Like natural processes that have existed on Earth for millennia, direct reliance on the sun frees us from efficiency as the central factor in our choices of energy conversion. Nature is notoriously inefficient when it comes to energy conversion: photosynthesis is only about 3 percent efficient, while human-made solar-to-hydrogen conversion is about 10–20 percent efficient (30 percent under concentrated illumination).6 Solar-to-fuels is about 12 percent. But nature wishes first and foremost to exist and accepts low efficiency as the cost of being. The sun provides approximately 1,000 watts per square meter to the Earth’s surface, or about as much energy in one hour as humanity uses per year.7 Under these circumstances, efficiency need no longer be the central concern.
Utqiaġvik (Barrow) in a storm, 2017. From the Utqiaġvik (Barrow) Sea Ice Webcam, operated by the University of Alaska Fairbanks
Process efficiency versus system efficiency
Here, we can make a distinction between process efficiency and system efficiency. The most efficient system does not necessarily consist of a set of maximally efficient processes. That is to say, less efficient processes may lead to system optimization, within realistic technological limits, by allowing the use of other key processes or beneficial choices at different critical stages. In the case of CCU, synthetic fuels would allow the continued use of existing infrastructure and technologies, like natural gas pipelines and internal combustion engines. Although both renewable hydrogen and electricity can be produced with fewer losses than synthetic fuels, these solutions have other disadvantages. Vehicles powered by fuel cells or batteries, for example, will require a renewal of the automobile fleet and fueling station infrastructure.
When considering these processes, the important question is: Which configuration produces the most efficient system? Life-cycle analysis is crucial, and so is keeping open the possibility of using an array of energy sources to achieve cross-regional integration. The solution to closing various biogeochemical processes will have to continually shift to optimally balance the available options.
CCU, like photosynthesis, has a multistep production chain, with total inefficiency dependent on the length of the chain. Beginning with PV cells and wind, the electricity generated by these sources can be stored in batteries or fed into the public grid. It can also be used immediately or stored as heat. The electricity can directly generate hydrogen, itself a fuel, or can be used in further reactions to create more complicated fuel molecules or precursor chemicals for industry.8 To give some specific examples: hydrogen can be combined with nitrogen, providing an emissions-free alternative to the Haber-Bosch process, or it can be combined with carbon (as well as oxygen and other atoms) to form any number of organic molecules such as methane, methanol, liquid organic hydrogen carriers (LOHCs), polyoxymethylene dimethyl ethers (OME, a diesel alternative), or even Michael Faraday’s bi-carburet of hydrogen. The carbon can come from fossil fuels or from carbon dioxide filtered out of the air via direct-air capture.9 With the latter technology, the carbon dioxide set free when fuels are burned can be collected and used anew in a circular process.
The CCU concept is not limited to the generation of carbon-neutral energy carriers. Carbon can be captured from flue gases—a by-product of industrial processes such as steel manufacturing—and cleaned, adjusted for constitution, and reused as precursors for the chemical industry or in the production of building materials (carbonates).10 The steel, concrete, and chemical industries are examples of sectors that cannot be decarbonized, because carbon chemistry is central to their production processes. However, like with synthetic fuels, the carbon need not come from newly obtained fossil sources—the carbon can instead be part of a closed loop. Current efforts focused on a one-time reuse of carbon in the chemical industry can result in up to a 50 percent reduction in carbon emissions.11 However, when recycling of plastics is widely established, a closed loop is certainly also possible. On the other hand, industrial emissions can also be used to manufacture fuels such as methanol, in which case the carbon cycle can be closed with current technology.
It’s important to note that the reuse of flue gases in the chemical industry is not a method of renewable energy storage and distribution, as is the case with synthetic fuels. Rather, it is an opportunity to gradually incorporate renewable energy and recycled CO2 into the production of steel and concrete and into the chemical industry at large. If renewable energy and hydrogen from renewable electricity are used in manufacturing processes, this form of CCU will be a fixture in our future combined sustainable consumption and circular economy landscapes.12
After harvesting and converting the sun’s energy into electricity with PV cells or wind turbines, we have many choices of what we can do with it. We may perform zero conversion steps and use the electricity directly, or we may choose many conversion steps—at the end of which we may again have electricity. We are free to choose the final form of energy suitable for any situation in which we may find ourselves. The inherent inefficiency of the process and practically boundless initial energy source that drives it frees us from having to define a maximally efficient energy conversion pathway. As long as the production chain begins with renewable electricity and uses carbon from the ambient atmosphere or industrial point sources, the carbon cycle remains closed and the question of efficiency need no longer be answered in the way we have become accustomed to.

Efficiency was the solution to fossil-fueled energy generation based on purely economic considerations. Now, however, we are faced with a two-dimensional problem of economic viability and environmental protection. Solving this problem requires a closure of the biogeochemical cycles to a degree that can be tracked, quantified, and even used as a marker for the Anthropocene. If a circular carbon economy is achieved, newly released carbon would not have the isotope fingerprint of either fossil carbon or the preindustrial atmosphere—rather, it would have a gradually stabilizing average of the two. As less and less new fossil carbon is emitted into the atmosphere, this average would result in a flattening of the Suess effect.
If, on the other hand, our efforts fail and the biogeochemical cycles are not closed, the Anthropocene may turn out to be a geological discontinuity rather than a geological age.
An expanded and revised version of this article was published as “The closed carbon cycle in a managed, stable Anthropocene” in The Anthropocene Review (2024): doi.org/10.1177/20530196231184777
Benjamin Johnson completed his PhD in physics in 2010 at the Technische Universität Berlin with a thesis on thin layer solar cells. His later research focused on catalytic materials for alternative fuels and the history of modern physics and chemistry. His approach combines the natural sciences with history and science communication to provide a roadmap for science-based governance.
Please cite as: Johnson, B. (2022). Closed Chemical Cycles. A Future Marker for the Anthropocene. In: Rosol C and Rispoli G (eds) Anthropogenic Markers: Stratigraphy and Context, Anthropocene Curriculum. Berlin: Max Planck Institute for the History of Science. DOI: 10.58049/ycvy-h654